e_RecyclingMetals
Recycling crucial metals using a bifunctional electrode
Rare-earth metals are indispensable in advanced/pioneering industries. On the other hand, rare-earth elements are scarce and unevenly distributed over the earth. Thus, in order to secure their stable supply, such rare-earth metals as Dy, Nd, and Pr must be recycled. Rare-earth element recovery and turning magnet scraps into possible alloys is an important issue. Against the above background, we proposed a novel molten salt electrochemical process to effectively recover the targeted rare-earth metal from scraps. The process’s principle is shown in Fig. 10.
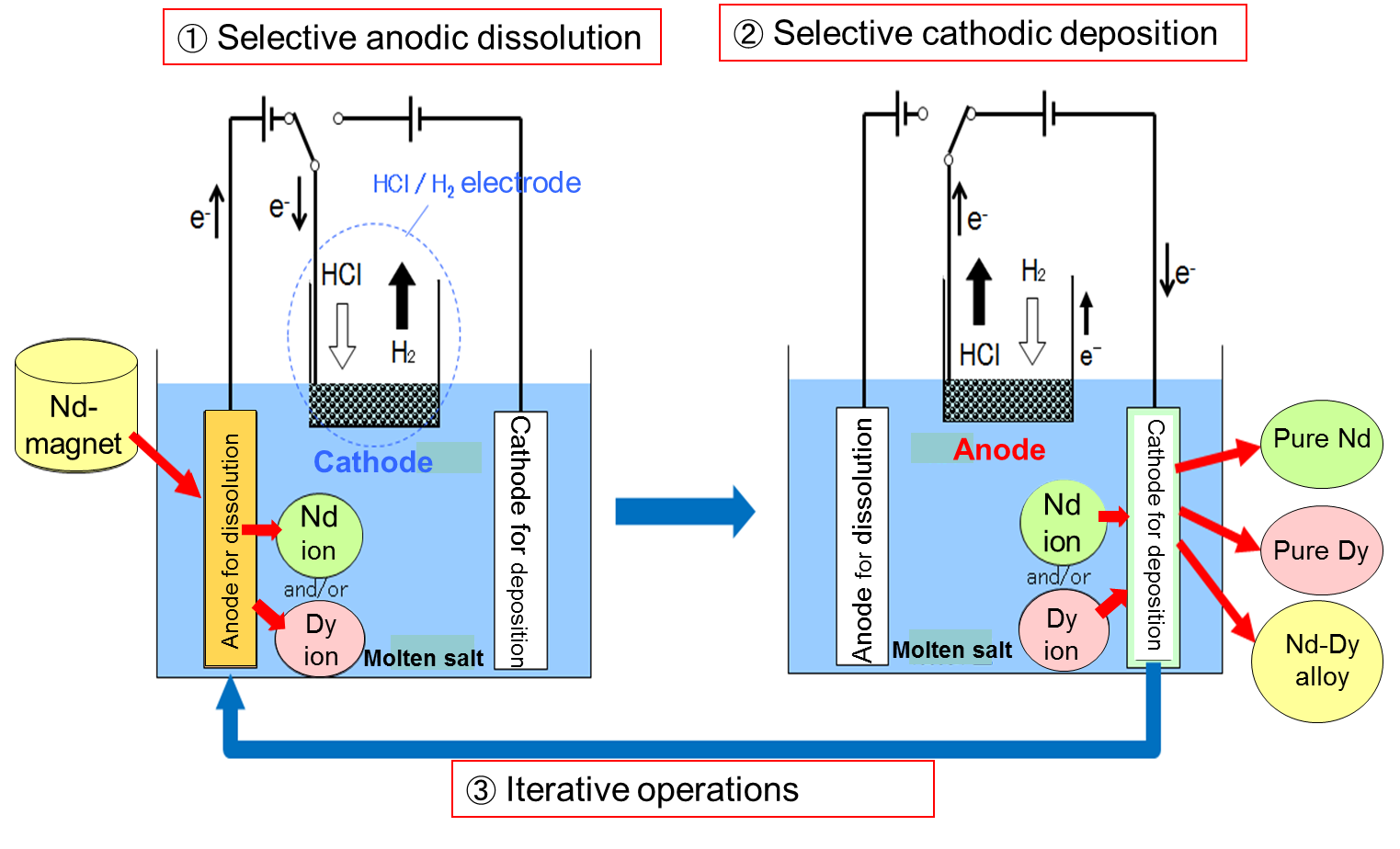
Fig. 10 Recycling process principle using a bifunctional electrode for HCl/H2 couple
Using electrochemical displantation (selective anodic dissolution) / implantation (selective cathodic deposition) reactions, for instance, we can efficiently recover Dy and Nd separately from a Dy-Nd containing magnet. In the process, as shown in Fig. 10, the 3rd electrode, which is termed a bifunctional electrode, functions as a cathode when a selective anodic dissolution reaction of an alloy proceeds and as an anode when a selective cathodic reaction proceeds on the working electrode. Note that sufficient difference exists between the alloy-forming potential of the Dy-Ni and Nd-Ni systems.
Fe and B remain in the 1st step of the anodic dissolution of the alloy (magnet) and are separately recovered as residue. When we want to obtain each metal with more separation efficiency, we can repeat the procedure (iterative operation), as shown in Fig. 10, until it achieves our final composition goal. The process can also be applied to the selective recovery of various crucial metals, including both precious and refractory metals. The combination of the process with the above plasma-induced discharge electrolysis is another attractive future R&D target.
